Confused about when a cloud component is a simple data system vs. a regulated medical device? This concise builder’s guide uses a decision tree, a quick “verb test,” and an EU mapping to help you design the right boundaries from day one.
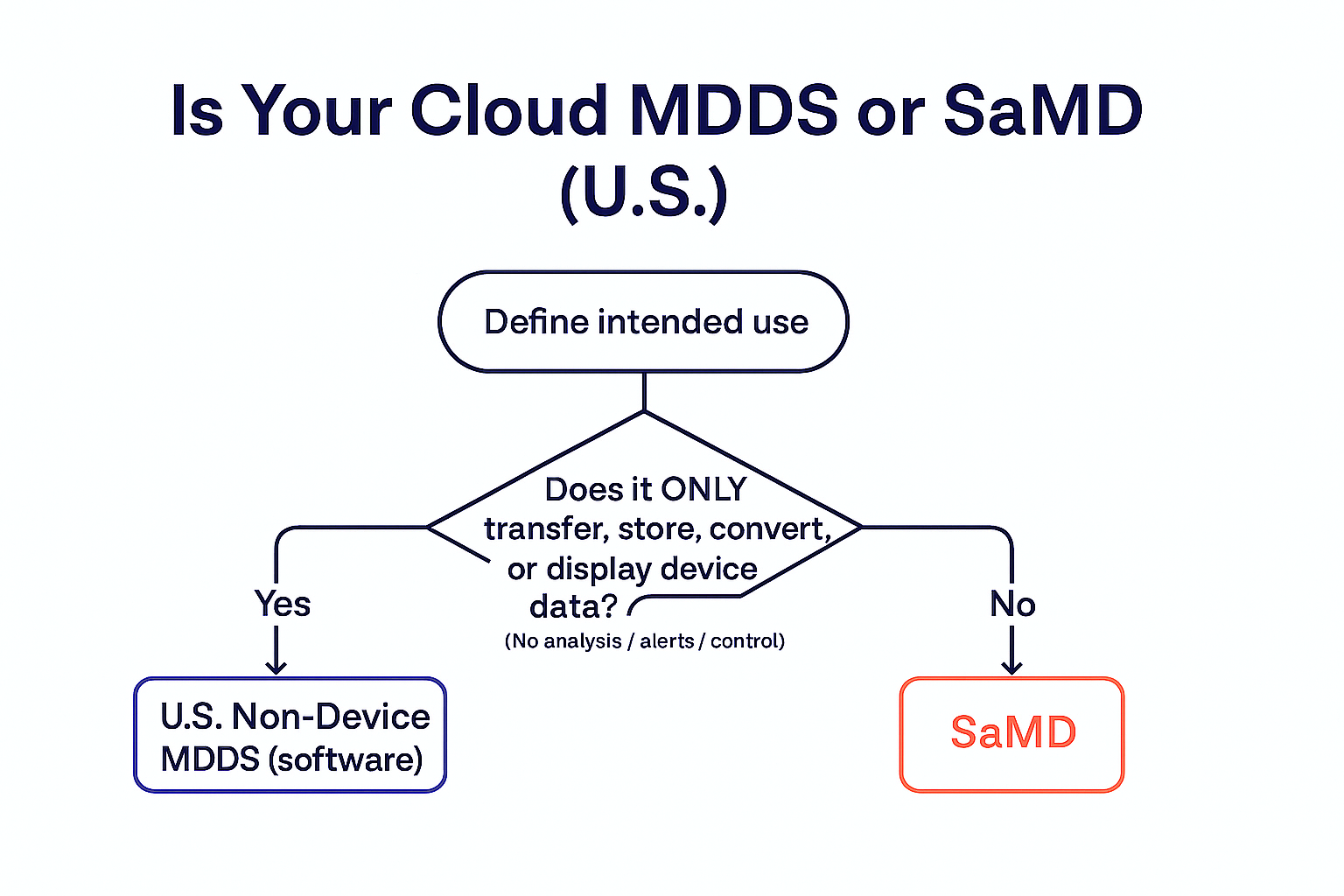
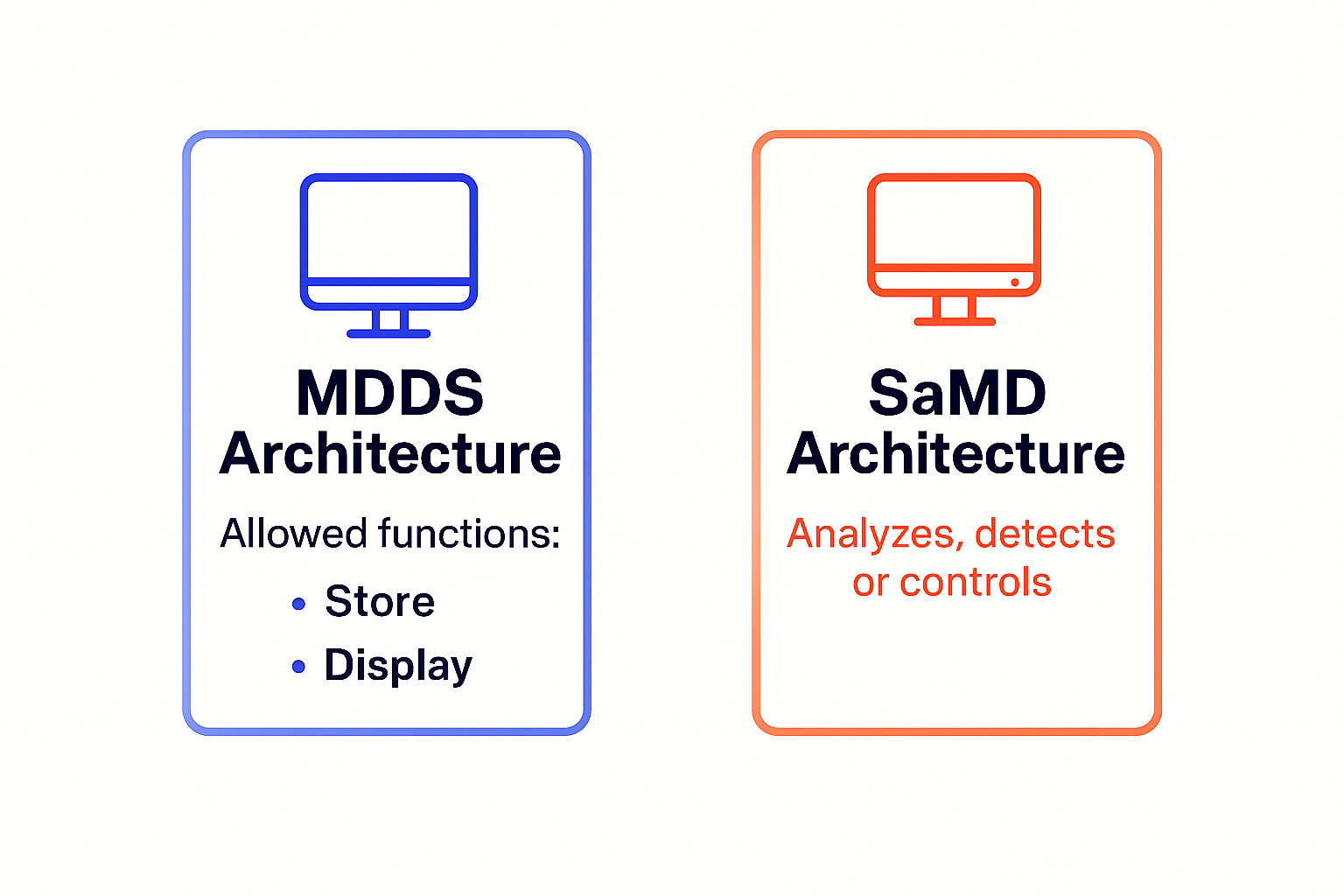
If your cloud only transfers, stores, converts format, or displays medical device data—and does not analyze, interpret, alarm/triage, or control devices—it likely fits U.S. Non-Device MDDS (software). Add analysis, alarms/triage, risk scoring, or device control and you’re in SaMD territory. For mixed products, separate functions and document their independence.
| Criteria | U.S. MDDS (Non-device software) |
U.S. SaMD (Device software) |
EU MDSW (Software under MDR) |
|---|---|---|---|
| Purpose | Move/format/display device data only | Performs a medical purpose independently | Qualifies as a medical device per MDR (e.g., diagnosis, prediction, monitoring, control) |
| Allowed functions | Transfer, store, convert format, display | Analyze, interpret, predict, triage/prioritize, alarm, control device | Depends on intended purpose; if only storage/display without medical purpose → typically not MDSW |
| Typical examples | Secondary display of ECG; FHIR pass-through | Arrhythmia detection; glucose hypoglycemia alerts; remote control | Software meeting Rule 11 with clinical claims; decision support that drives action |
| Regulatory pathway | Non-device (no 510(k)); labeling must reflect MDDS scope | Device pathway (510(k)/De Novo/PMA) | CE marking under MDR (classification via Rule 11, Notified Body where applicable) |
| What triggers device status? | Any analysis/interpretation, alarms/triage, risk scores, or device control | Already device | Medical purpose + classification rules; storage/display alone without medical purpose is out of scope |
| Documentation focus | Clear intended use & boundaries; marketing copy control | Safety case, clinical evaluation, software lifecycle, cybersecurity | General safety & performance, clinical evaluation, PMS, cybersecurity |
PCCP = Predetermined Change Control Plan. For AI/ML or frequently updated SaMD, PCCP helps predefine what can change and how you’ll validate it.
Architecture choices and wording choices are regulatory choices. Decide early whether your cloud is MDDS-only or SaMD—and design the boundaries accordingly. If you’re exploring a PCCP for frequent updates, let’s talk about how BioT keeps the evidence tidy.
Disclaimer: This article is general guidance, not legal advice. For specific products, consider FDA’s Q-Submission process or consult your Notified Body.